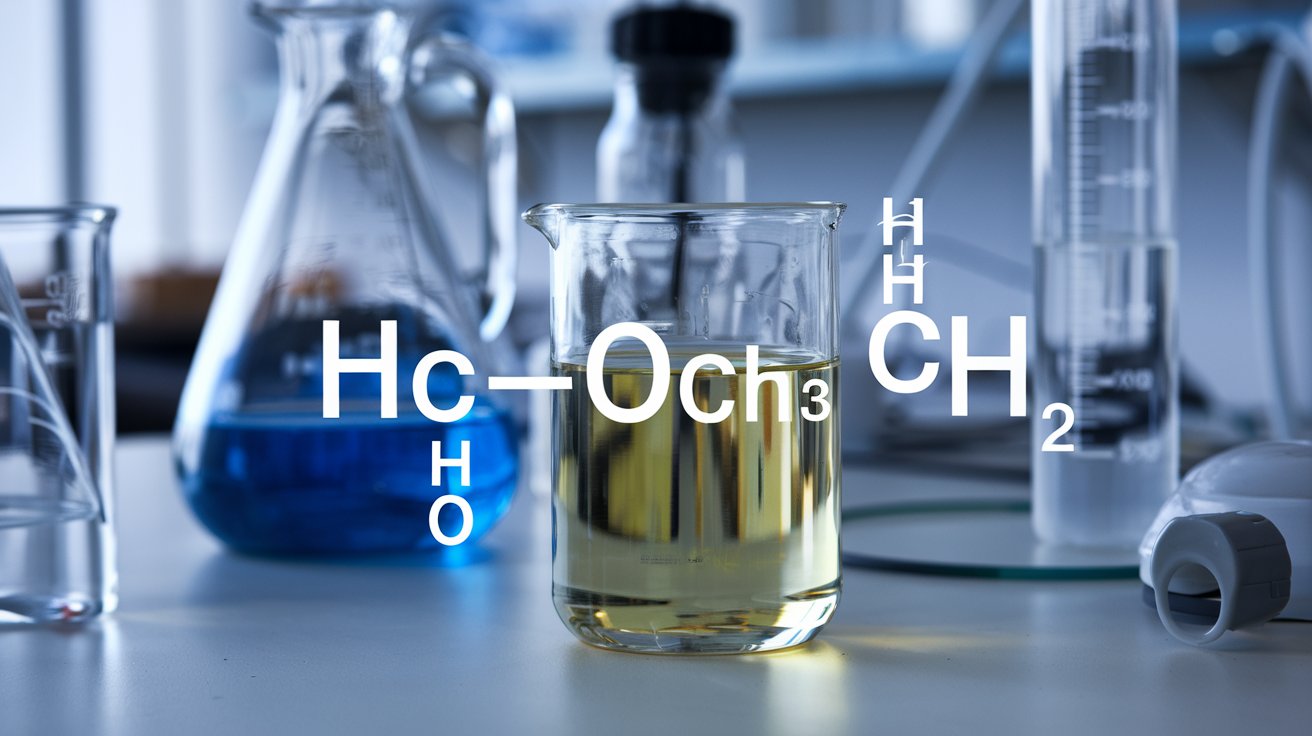
hcooch ch2 h2o may look like a jumble of letters and numbers, but it represents something important in chemistry that affects many things around us. When you break it down, it shows how methyl formate (HCOOCH₃), a methylene component (CH₂), and water (H₂O) can interact. These parts can combine in different ways, especially in a process called hydrolysis, where water helps turn methyl formate into formic acid and methanol. This reaction is used in labs and industries to make substances that are used in cleaning, dyes, or even in fuel technology.
Understanding hcooch ch2 h2o helps you see how small molecules can do big work. Water isn’t just there to look pretty—it often makes or breaks chemical bonds. The CH₂ part acts as a connector in bigger molecules, while methyl formate is an ester that water can split. So, hcooch ch2 h2o is not just a formula, it explains many chemical changes. Whether you are studying chemistry or curious about why cleaners smell a certain way or why some fuels work better, knowing about this helps.
What is hcooch ch2 h2o? Breaking Down the Parts
hcooch ch2 h2o may look like a tricky formula, but it is easy to understand if we break it down. hcooch stands for methyl formate, which is a type of ester. ch2 is a simple group that connects carbon atoms in many molecules. h2o is just water, which you already know well. When these three meet, they can take part in a process called hydrolysis. This simply means that water helps to split methyl formate into other smaller molecules like methanol and formic acid. Scientists study this because it is very useful in making many everyday products. From perfumes to cleaners, this small formula plays a big role. Knowing about hcooch ch2 h2o helps us see how tiny molecules can make huge changes in chemistry and in the world around us.
How hcooch ch2 h2o Reaction (Hydrolysis) Actually Works
The reaction of hcooch ch2 h2o is called hydrolysis, and it works in a very cool way. Water acts like a helper that breaks the bond in methyl formate. When this happens, the molecule splits into formic acid and methanol. Formic acid is useful in making preservatives, leather products, and even in farming. Methanol can be used as a fuel or solvent. The process happens faster when heat or a catalyst is added. Catalysts can be acids or bases that speed up the change without being used up. This is why industries use this reaction to make products quickly and safely. Learning about hcooch ch2 h2o shows us how chemistry uses simple building blocks to create useful things. It is a great example of how water and other molecules work together.
Role of Water (H₂O) in the hcooch ch2 h2o Process
Water is the main hero in the hcooch ch2 h2o reaction. Without water, there would be no hydrolysis at all. Water molecules attack the bond in methyl formate and break it apart. This action creates new products like methanol and formic acid. It may sound fancy, but water is simply doing what it does best—helping molecules change shape. This is why many chemical reactions need water. In labs, scientists control the amount of water to get the right results. If there is too little water, the reaction will be very slow. If there is too much, the products may get diluted. Understanding how water works in hcooch ch2 h2o helps us respect how powerful such a simple molecule can be. It is the quiet worker behind many reactions we rely on every day.
Uses of hcooch ch2 h2o in Industry and Everyday Life
hcooch ch2 h2o may sound like a lab thing, but it has many uses in daily life. The products made from this reaction, like methanol and formic acid, are found in many industries. Methanol is used as a fuel, a solvent for paints, and even in making plastics. Formic acid is used in food preservatives, animal feed, and leather tanning. These uses make the reaction very important for industries that create products we use every day. Without hcooch ch2 h2o, many manufacturing processes would be slower or more expensive. This is why scientists keep studying this reaction and finding better ways to control it. When you think about hcooch ch2 h2o, you are really thinking about many products that make modern life easier, cleaner, and more efficient.

Safety Tips When Working With hcooch ch2 h2o Components
Safety is very important when working with hcooch ch2 h2o components like methyl formate and methanol. These substances can be harmful if handled without care. Methyl formate has a strong smell and can irritate your nose or throat. Methanol is toxic and should never be touched or swallowed. Always wear gloves, goggles, and a mask when working with these chemicals in a lab. Make sure there is good airflow to avoid breathing too many fumes. Store them in safe, closed containers away from heat and flames. Water is safe, but when mixed with these chemicals, reactions can release heat, so always be careful. Following safety tips keeps you and others safe. It also helps avoid accidents that can harm the environment. Chemistry is exciting, but safety comes first.
Common Misunderstandings About hcooch ch2 h2o
Many people think hcooch ch2 h2o is too hard to understand or only for scientists, but that is not true. It is simply a way to show how molecules interact with each other. Another misunderstanding is that this reaction is dangerous by itself. It is not dangerous if done in a controlled way with the right safety steps. Some people also think this formula is only for making fuel, but it is used in many industries including food and farming. Clearing up these myths helps more people learn about the beauty of chemistry. When we understand hcooch ch2 h2o better, we can see how science helps create products that improve our daily lives. It makes chemistry less scary and more exciting. Knowledge is the key to seeing chemistry as a friend, not something to fear.
Why Scientists and Engineers Care About hcooch ch2 h2o
Scientists and engineers spend time studying hcooch ch2 h2o because it is very useful in real life. They want to make the reaction faster, cheaper, and safer so industries can make better products. Engineers look for new machines that can do this reaction without wasting energy. Chemists look for new catalysts that make it happen more quickly. When these experts work together, we get new solutions that help the environment and save money. This is why research on hcooch ch2 h2o is so active. Every discovery can make industries work better and make products that are safer for people. The study of this simple formula shows how small things lead to big changes in science and technology. It is a perfect example of how chemistry improves the world around us.
Conclusion
hcooch ch2 h2o may look like a long, confusing formula, but it is really about how small molecules work together. Water, methyl formate, and CH2 connect and change to make useful things like methanol and formic acid. This reaction happens in labs and industries, helping make fuel, cleaners, and food products.
Learning about hcooch ch2 h2o shows us that chemistry is not scary. Even small molecules can do big jobs. By understanding them, we can see how science makes life easier and safer. This simple formula teaches us how tiny things create big changes in the world around us.
FAQs
A: It is a formula showing methyl formate, CH2, and water working together in a chemical reaction.
A: Water splits methyl formate into methanol and formic acid through hydrolysis.
A: It is used in industries to make fuel, cleaners, plastics, and food preservatives.
A: Yes, if handled carefully with gloves, goggles, and good ventilation.
A: To make reactions faster, safer, and more useful for everyday products.
This Article Prouded Presented By matchstats.blog